Overview
Purpose of the Clinical Reporting Component
This component of the handbook describes the supporting Current Procedural Terminology (CPT®)[1] content available as clinical reporting guidance. Material covered includes information about the CPT content directed at clinical reporting and benefits of its use. This component also describes approaches for implementing clinical reporting guidance and how clinical reporting guidance may improve CPT content reporting. An example of a clinical reporting guidance policy is included.
Intended Audiences
This portion of the education handbook is relevant to all audiences, including
- Physicians/doctors
- Physician specialist groups
- Health service programs – quality improvement programs
- Health service delivery organizations
- Vendors of EMRs, EHRs, and specific clinical and functional areas
- Research organizations
- Local/national governments
- Local/national physician/doctor organizations/associations
- National information/standards and policy organizations
- Health information management professionals
- Those responsible for collecting service and procedure data
- Analysts
Learning Objectives
After reviewing the content in the Clinical Reporting Component, the reader is expected to be able to:
- State what comprises clinical reporting guidance.
- Identify where within the CPT content clinical reporting guidance is found.
- Name a benefit of applying clinical reporting guidance to reporting CPT content.
- Describe approaches to implementation of clinical reporting guidance.
- Explain how the use of clinical reporting guidance has the potential to improve CPT content reporting.
- Give an example of a clinical reporting guidance policy.
Modules
The Clinical Reporting Component includes two modules:
Module One: Overview of Clinical Reporting Guidance – Describes what constitutes clinical reporting guidance within the CPT® content and states the benefits of applying the guidance.
Module Two: Implementation of Clinical Reporting Guidance – Informs the reader of approaches to implementing clinical reporting guidance. This module also illustrates how clinical reporting guidance has the potential to improve CPT content reporting. Included is an example of clinical reporting guidance policy.
Each module is structured as follows. Note that some sections may not apply to a module.
Introduction – An introduction to the topic being discussed and why it is important.
- Intended Audience – The intended audience for the module.
- Learning Objectives – A list of learning objectives that will be covered in the module.
Main Module Content – The main content may consist of five main sub-sections.
- Personnel – People who are involved in the topic.
- Tooling – Tooling and requirements that are required to apply the approach.
- Approach – The approach or methodology specific to a topic.
- Process – The interaction between personnel with other personnel and/or tooling.
- Challenges – Challenges associated with applying the approach.
Practical Use Example – An example of how the approach has been used.
MODULE ONE: Overview of Clinical Reporting Guidance
Introduction
This module describes what constitutes clinical reporting guidance within the CPT® content, where the guidance is found, and states the benefits of applying the guidance.
Intended Audiences
As noted above in the Component Overview.
Learning Objectives
After reviewing the content in this module, the reader is expected to be able to:
- State what comprises clinical reporting guidance.
- Identify where within the CPT content clinical reporting guidance is found.
- Name a benefit of applying clinical reporting guidance to reporting CPT content.
Overview of Clinical Reporting Guidance
Clinical reporting guidance consists of the guidelines and parenthetical notes (called parentheticals) found within the CPT content, which provide instructions on how to accurately report a procedure or service. While comprehensive, the guidance is intended to prevent errors of significant probability and is not all inclusive.
CPT guidelines are instructions, such as definitions, that provide clinical reporting information on a block of CPT content. Each section of CPT content has a set of guidelines as do many of the headings. For example, the following guidelines are found in the Surgery section under the Musculoskeletal System and Endoscopy/Arthroscopy headings:
- Surgical endoscopy/arthroscopy always includes a diagnostic endoscopy/arthroscopy.
- When arthroscopy is performed in conjunction with arthrotomy, add modifier 51.
Parenthetical notes are instructions that verify the intent of the code(s). The notes are enclosed in parentheses within the CPT content and may be found preceding or following a code listing and within a code descriptor.
For example, the parenthetical notes listed under the CPT code listing for arthroscopic (endoscopic) capsulorrhaphy are:
- (For open procedure, see 23450-23466)
- (To report thermal capsulorrhaphy, use 29999)
An example of a parenthetical note within a code descriptor is:
- 29824 Arthroscopy, shoulder, surgical; distal claviculectomy including distal articular surface
(Mumford procedure)
Some user benefits of applying clinical reporting guidance include:
- Improved accuracy and quality of clinical reporting by clinicians.
- Prevention of errors prior to submission to an external organization, such as a claims administrator, resulting in fewer claim questions and denials.
- Better quality clinician data for researchers and analysts.
Personnel
The clinical reporting guidance is for all users of CPT® content. For example, an electronic medical record (EMR) vendor may provide clinical reporting guidance as a prompt within a clinical documentation application for a clinician to consider when selecting a CPT descriptor.
Tooling
CPT® Link is a suite of files that contains the content from the CPT® Professional Edition book in both human-readable and machine-processable formats. The human-readable formats are in the form of PDFs and a
web-based representation of the codebook and is intended for all users while the machine-processable formats are primarily intended for use in software applications.
Included in CPT® Link are guidelines and parentheticals content files, and files that can be used to validate the guidelines and parentheticals. For example, the Report With file is a machine-processable file that corresponds to CPT parenthetical notes when multiple CPT codes for specified procedures must be reported together. See the CPT® Link Component for additional information.
Practical Use Example: CPT® Link Data Files
Edits
The Report With and Exclude files are machine-processable files that correspond to CPT content parentheticals for when a CPT code for a specified procedure must or must not be reported in conjunction with another CPT code.
Application example: Programming of the rules into EMRs for data quality and/or claims engine for adjudication.
The following are sample rows from the Report With file. The CPT code in Code 1 is for an add-on procedure, which must always be reported with the CPT code in Code 2, for the primary procedure.
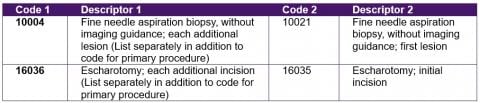
Extracts
The Coding Tips and Citations files contain all the coding tips found throughout the CPT® Professional Edition book and other reference documents where a CPT code for a specified procedure is cited.
Application example: Utilizing as clinical data quality checks to the assigned codes.
The following are sample rows from the Coding Tips file. The coding tips are for CPT code 45333 – Sigmoidoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps.

MODULE TWO: Implementation of Clinical Reporting Guidance
Introduction
This module informs the reader of approaches to implementation of clinical reporting guidance and illustrates how clinical reporting guidance may improve CPT® content reporting. An example of a clinical reporting guidance policy is provided.
Intended Audiences
As noted above in the Component Overview.
Learning Objectives
After reviewing the content in this Clinical Reporting Component, the reader is expected to be able to:
- Describe approaches to implementation of clinical reporting guidance.
- Explain how the use of clinical reporting guidance has the potential to improve CPT content reporting.
- Give an example of a clinical reporting guidance policy.
Implementation of Clinical Reporting Guidance
The use of CPT content guidance improves CPT content reporting by minimizing improper clinical data collection at the time of capture and facilitating data integrity for secondary use. With clinical reporting guidance available early in the data flow, more consistent and standardized data capture results in fewer errors downstream and less time spent investigating the cause and correcting of a clinical reporting problem. Audit risks lessen with established standard clinical reporting practices that produce high-quality, reliable data. Clinician reporting variability declines thereby ensuring data uniformity for clinical research, registries, and quality improvement. Data validation edits based on clinical reporting guidance can also streamline a claims administrator workflow by identifying red flags in need of correction before adjudication.
Personnel
The following personnel have a role in implementing clinical reporting guidance.
- Clinicians
- Coding professionals
- EMR, EHR, HeIS, or HIS vendors
- Claims administrators
- Research organizations
- Physician/doctor organizations/associations
- Governmental policy analysts
- Governmental data analysts
Tooling
Vendor and provider applications found in EMR, EHR, HeIS, HIS, or billing and claims adjudication systems.
Approach
Two approaches to implementation of clinical reporting guidance are described below.
Local/national government approach
A local/national government approach to implementation of clinical reporting guidance in conjunction with CPT content adoption involves:
- Review current program policy and define program policy content blocks
- Consider all program clinical information use
- Align clinical reporting requirements with CPT content structure and processes
- Agree to priority areas to start/test approach
- Develop documentation for each priority area
- Gather all background documents/analyses
- Assess current policy, determine what remains, and place into broad subject blocks
- Analyze CPT content clinical reporting guidance and CPT resources (see the Educational Materials Component)
- Evaluate what is similar to current policy and what is different from current policy
- Describe the outcome of the analysis
- List any outstanding items requiring an impact analysis
- Revise current policy and develop new content where gaps exist
- Construct the recommended policy and its rules
- Determine what new content is needed and what part of current policy should remain
- Revise current policy and develop new content where gaps exist
- Format policy as per template
- Policy Topic
- Policy number
- Date of origin
- Related policies
- Date last updated
- Date last reviewed
- Instructions for Use
- Application
- Overview
- Definition
- Clinical Reporting Requirements
- Questions and Answers
- Attachment
- References
- Policy History/Revision Information
- Clinical Reporting Policy Rules
- Review outcome of test approach with CPT® Content Program Policy and Implementation Team
- Revise approach as needed based on feedback
- Define topic priority order and schedule
- Use current policy content blocks and frequently reported procedures (top 90%) as the basis for policy topic development
- Assume concurrent topic analysis and policy development completion
- Proceed with completion of clinical reporting policy
- Review by the CPT Content Program Policy and Implementation Team and dissemination to local/national government for approval
- Local/national government approves
- Clinical reporting policy integrated into the overall program policy
Vendor approach
A vendor approach to implementation of clinical reporting guidance in an electronic system involves the use of certain CPT content found in CPT® Link and the CPT Standard Data files. The implementation of CPT content guidance can be accelerated with these computer-executable data files. For example,

Defining the technical functional requirements to ensure the terminology is implemented correctly and consistently is also part of the approach. Two examples of minimum requirements for data capture (DC) are:
DC1 - Systems MUST validate CPT codes for specified procedures and modifiers (where applicable) against the CPT guidelines
Systems MUST have functions in place to validate CPT codes for specified procedures (and modifiers, where applicable) when they are entered into a patient record. Systems MUST also display reasons why a CPT code for a specified procedure (and modifiers, where applicable) is rejected. Providing the reasons why the use of a CPT code for a specified procedure and/or modifier is rejected can help educate users on how to correctly report procedures rendered.
The main types of guideline (rules) validations are:
- Ensure an add-on procedure is used in conjunction with a primary procedure
- Ensure two or more CPT codes for specified procedures can be reported in conjunction with each other
- Ensure modifiers can be used with a CPT code for a specified procedure
DC2 - Systems SHOULD display additional information for CPT® codes for specified procedures
Additional information about a CPT descriptor SHOULD be available in the form of tooltips, dialog boxes, or popups. This can include the CPT code, Long Descriptor, guidelines (rules), and units. While this is not a mandatory requirement, the additional information may be useful for users when they are choosing a procedure to report.
For example, when the cursor is placed over the CPT descriptor, “Fine needle aspiration biopsy using computed tomography (CT) guidance for each additional lesion,” in a list of search results, the following information should be displayed:

Process
There are a number of possible interactions when using clinical reporting guidance. For example,
EMR, EHR, HeIS, or HIS vendors – Integrate the CPT® Link data files containing the computer-executable guidelines and parentheticals content files into their health information system application.
Claims administrators – Apply the CPT® Link data files containing the computer-executable guidelines and parentheticals content files as edits to assess the appropriateness of clinical reporting.
Clinicians – Address an EMR, EHR, HeIS, or HIS prompt when selecting a procedure based on CPT content clinical reporting guidance.
Coding professionals – Use the clinical reporting guidance to determine the correct procedure for the clinician to report.
Research organizations – Draw upon the clinical reporting guidance when conducting analysis of CPT content found in databases.
Physician/doctor organizations/associations – Utilize the clinical reporting guidance to educate membership on the procedural intent to improve the physician/doctor data.
Governmental policy analysts – Use the clinical reporting guidance to determine where interpretation exists in order to strengthen the clinical reporting policy.
Governmental data analysts – Consider the clinical reporting guidance when studying physician data to uncover any variations due to interpretation.
Challenges
A potential challenge in the assessment of current program policy is separating the content pertaining to clinical reporting from other uses, such as the basis for reimbursement. For example, the current policy stated below contains both clinical reporting and billing guidance.
Prolonged Consultation: A prolonged consultation may be applied to cases where the consultation extends beyond one hour for comprehensive consultations. A prolonged consultation cannot be claimed with a limited consultation. Prolonged consultations are paid in 15-minute time blocks or portion thereof.
Another challenge occurs when attempting to write new policy content that strictly addresses clinical reporting only and does not include descriptions of other uses for CPT® content.
Practical Use Example: Clinical Reporting Policy for Add-On Procedures
Add-On Procedures Policy
- Policy Number: XX.XX
- Related Policies: XX.XX
- Date of Origin: YYYYMMDD
- Last Updated: YYYYMMDD
- Last Reviewed: YYYYMMDD
Instructions for use
The procedures described in local/national government policies are subject to the terms, conditions, and limitations of the overall program. Clinicians are responsible for accurately, completely, and legibly documenting the procedures rendered.
Application
This policy applies to all clinicians regardless of compensation model.
Overview
The CPT® codes for add-on procedures are required by local/national governments for clinical reporting.
There are several designations of add-on procedures based on CPT content information. CPT content designates these additional or supplemental procedures with the + symbol within the CPT code listing and in Appendix D of the CPT Professional Edition book.
Definition
Per CPT content, some procedures are commonly carried out in addition to the primary procedure performed. Specific descriptor nomenclature that includes phrases such as "each additional" or "(List separately in addition to primary procedure)", or "done at the time of other major procedure" is also part of the add-on procedures.
Add-on procedures are a specific type of supplemental procedure describing additional intra-service work associated with the primary procedure performed; eg, additional digit(s), lesion(s), neurorrhaphy(ies), vertebral segment(s), tendon(s), joint(s), vaccine(s), minute(s), hour(s), etc.
Add-on procedures can be found in many sections of CPT content, not just the Surgery section.
Clinical reporting requirements
The primary procedure and the add-on procedure form a pair of connected services. The primary procedure may also sometimes be referred to as the "parent procedure" for the add-on procedure. Add-on procedures may not be reported without an accompanying primary procedure; when this occurs, the add-on procedure is considered an "orphan procedure," which has been incorrectly reported.
Report only a procedure identified as an add-on when the same physician /doctor has performed the primary procedure at the same surgical session or patient encounter. If the same physician/doctor has not performed the primary procedure, then do not report the add-on procedure.
Questions and answers
Question 1: Is there any exception to the requirement that an add-on procedure may not be reported without an accompanying primary procedure?
Currently there is only one exception. A physician/doctor may report CPT® code 99292 – Critical care, evaluation and management of the critically ill or critically injured patient; each additional 30 minutes (List separately in addition to code for primary service) without its primary procedure CPT code 99291 – Critical care, evaluation and management of the critically ill or critically injured patient; first 30-74 minutes if two or more physicians of the same specialty in a group practice provide critical care services to the same patient on the same date of service.
For the same date of service only one physician of the same specialty in the group practice may report CPT® code 99291 with or without CPT code 99292, and the other physician(s)/doctor(s) must report their critical care services with CPT code 99292.
Question 2: A procedure I reported in the past with another procedure does not have a + symbol. How do I make sure there is no loss of clinical content when reporting these situations?
With a changeover to CPT content for clinical reporting, a single code may be available where in the past multiple codes were necessary to clinically report the procedure(s). Review CPT descriptors and reporting requirements to ensure accurate clinical reporting. For example, CPT content defines a colonoscopy as the examination of the entire colon from the rectum to the cecum and may include the examination of the terminal ileum. In the case of a colonoscopy of the descending, transverse, and ascending colon including the cecum with biopsy(ies), one would report CPT code 45380 – Colonoscopy, flexible; with biopsy, single or multiple. If the colonoscopy is performed and does not reach the cecum, report colonoscopy procedure CPT code 45380 with modifier 52 (reduced services) and provide appropriate documentation.
Attachment
None
References
CPT® 2020
Policy history/revision information
Policy developed YYYYMMDD
Clinical reporting policy rules for add-on procedures
The clinical reporting rules for add-on procedures are:
- An add-on procedure without an accompanying primary procedure should not be reported with the current exception of a procedure reported under CPT code 99292, which is used when two or more physicians of the same specialty in a group practice provide critical care services to the same patient on the same date of service.
- Modifier 51 (multiple procedures) should not be reported with an add-on procedure.

NAVIGATION
Component 3: Assessment of Feasibility
Component 7: Clinical Reporting
Component 9: Technical Requirements
Component 10: Educational Material